COVID-19
Latest Insights & Products for SARS-CoV-2/COVID-19 Research
Go to:
>> Coronaviruses (CoVs) - SARS-CoV & SARS-CoV-2 [2019-nCoV]
>> ACE2 & Functional Receptor for SARS Coronaviruses
>> Biological Therapeutic Strategies against SARS-CoV-2 and COVID-19
>> Cytokine Storm and Severity of COVID-19
>> Overview on the AdipoGen Life Sciences Panel of Products
Coronaviruses (CoVs) - SARS-CoV & SARS-CoV-2 [2019-nCoV]
Coronaviruses (CoVs) are enveloped non-segmented positive-sense RNA viruses infecting human and vertebrates. They are classified into four types (genus), α-CoV, β-CoV, γ-CoV and δ-CoV. They can infect the respiratory, gastrointestinal, hepatic and central nervous system of human and many wild animals. The family of Coronaviridae constantly circulates within the human population and mainly causes mild respiratory diseases. Recently, a new severe acute respiratory syndrome β-coronavirus called SARS-CoV-2 (or 2019-nCoV) has emerged, which causes an epidemic of acute respiratory syndrome called coronavirus human disease 2019 or COVID-19. Typical clinical symptoms of these patients are dry cough, fever, breathing difficulties, headache and pneumonia. Disease onset may result in progressive respiratory failure, heart tissue damage and even death.
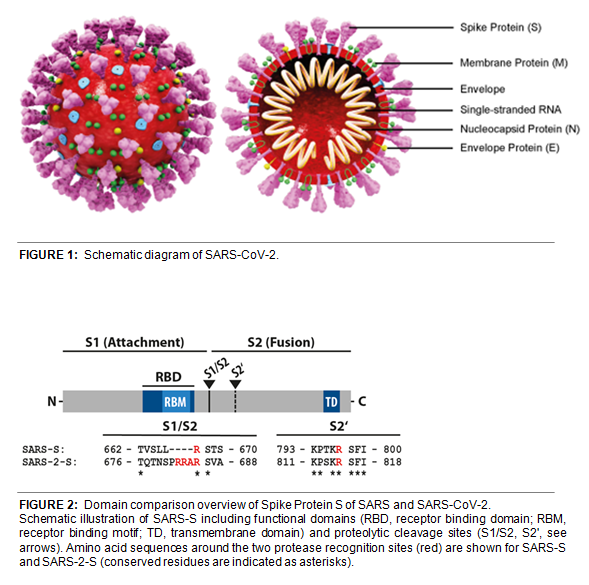 SARS-CoV-2 shares 79.5% sequence identity with SARS-CoV and is 96.2% identical at the genome level to the bat coronavirus BatCoV RaTG133, suggesting it had originated in bats. The coronaviral genome encodes four major structural proteins: the Spike (S) protein, Nucleocapsid (N) protein, Membrane/Matrix (M) protein and the Envelope (E) protein (see Figure 1). The SARS Envelope (E) protein plays a role in viral budding and virion envelope morphogenesis. The SARS Membrane/Matrix (M) protein is one of the major structural viral proteins. It is an integral membrane protein involved in the budding of the viral particles and interacts with SARS Spike (S) protein and the Nucleocapsid (N) protein. The N protein contains two domains, both of them bind the virus RNA genome via different mechanisms.
SARS-CoV-2 shares 79.5% sequence identity with SARS-CoV and is 96.2% identical at the genome level to the bat coronavirus BatCoV RaTG133, suggesting it had originated in bats. The coronaviral genome encodes four major structural proteins: the Spike (S) protein, Nucleocapsid (N) protein, Membrane/Matrix (M) protein and the Envelope (E) protein (see Figure 1). The SARS Envelope (E) protein plays a role in viral budding and virion envelope morphogenesis. The SARS Membrane/Matrix (M) protein is one of the major structural viral proteins. It is an integral membrane protein involved in the budding of the viral particles and interacts with SARS Spike (S) protein and the Nucleocapsid (N) protein. The N protein contains two domains, both of them bind the virus RNA genome via different mechanisms.
The CoV Spike (S) protein assembles as trimer and plays the most important role in viral attachment, fusion and entry. It is composed of a short intracellular tail, a transmembrane anchor, and a large ectodomain that consists of a receptor binding S1 subunit and a membrane-fusing S2 subunit (see Figure 2, adapted from Cell, Hofmann, et al. (2020)). The S1 subunit contains a receptor binding domain (RBD), which binds to the cell surface receptor angiotensin-converting enzyme 2 (ACE2) present at the surface of epithelial cells.
LITERATURE REFERENCES:
- The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status: Y.R. Guo, et al.; Mil. Med. Res. 7, 11 (2020) (Review)
- SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor: M. Hoffmann, et al.; Cell (Epub ahead of print) (2020)
- Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine: W. Tai, et al.; Cell Mol. Immunol. (Epub ahead of print) (2020)
- Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2: R. Yan, et al.; Science 367, 1444 (2020)
- Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein: A.C. Walls, et al.; Cell (Epub ahead of print) (2020) (Review)
ACE2 & Functional Receptor for SARS Coronaviruses
 Angiotensin-converting enzyme 2 (ACE2) is a type I transmembrane metallocarboxypeptidase within the renin-angiotensin system (RAS), which plays a key role in blood pressure regulation, fluid and electrolyte balance, thirst, cardiac/renal function and growth. ACE2 is expressed on the cell surface of type 2 alveolar epithelial cells in the lungs as well as on cells in many other tissues. ACE2 shares approximately 60% homology with ACE, the other key enzyme of the RAS system.
Angiotensin-converting enzyme 2 (ACE2) is a type I transmembrane metallocarboxypeptidase within the renin-angiotensin system (RAS), which plays a key role in blood pressure regulation, fluid and electrolyte balance, thirst, cardiac/renal function and growth. ACE2 is expressed on the cell surface of type 2 alveolar epithelial cells in the lungs as well as on cells in many other tissues. ACE2 shares approximately 60% homology with ACE, the other key enzyme of the RAS system.
ACE2 converts angiotensin II (Ang II) into Ang(1–7), which acts on the Mas receptor and plays a role in cardiovascular disease to lower blood pressure through vasodilation and by promoting kidney sodium and water excretion, but also to lower inflammation. The effects of ACE2 directly oppose those induced by ACE–Ang II signaling, whereby ACE converts Ang I into Ang II, which increases blood pressure by inducing vasoconstriction, increasing kidney reabsorption of sodium and water and promoting inflammation.
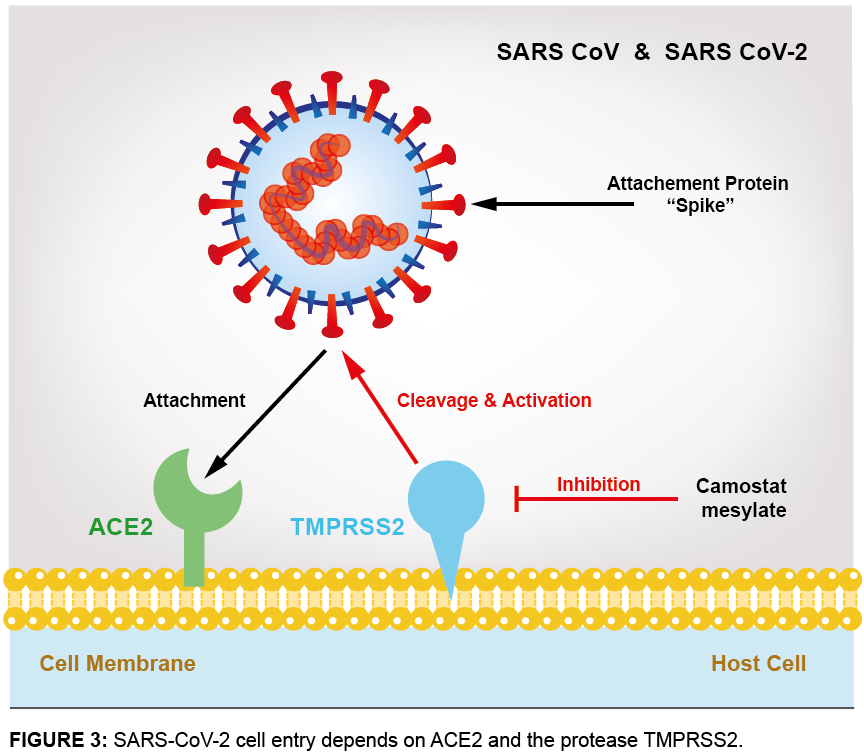
ACE2 has been identified as a key receptor on target cells for SARS-CoV infections in 2002. ACE2 functions as the entry receptor of the new SARS-CoV-2 coronavirus that emerged in China in 2019 and is the cause of the new disease COVID-19. Strong binding of the spike protein of SARS-CoV-2 to ACE2, along with proteolytic cleavage of ACE2 by transmembrane serine protease 2 (TMPRSS2), facilitates entry of the virus into cells, viral replication and cell-to-cell transmission. The spike protein priming by the co-receptor serine protease TMPRSS2 is crucial for SARS-CoV-2 infection of target cells and spread of the coronavirus throughout the host (see Figure 3).
LITERATURE REFERENCES:
- Angiotensin Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: M. Gheblawi, et al.; Circ. Res. (Epub ahead of print) (2020)
- SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor: M. Hoffmann, et al.; Cell (Epub ahead of print) (2020)
Biological Therapeutic Strategies against SARS-CoV-2 and COVID-19
18 years ago the SARS-CoV lead to respiratory diseases in infected people. To date there are no effective vaccines against any type of the coronavirus, which can cause pneumonia and possibly bronchitis. Nevertheless, there are several strategies that are being pursued.
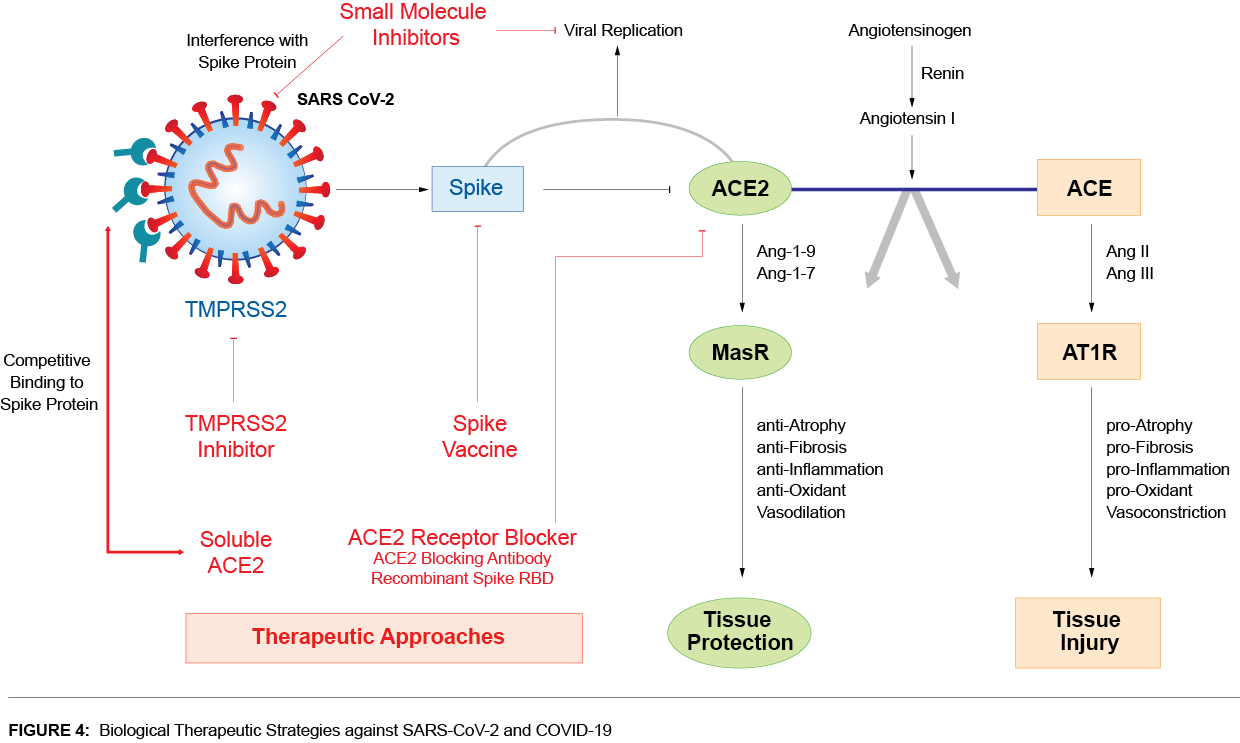
Soluble Human ACE2:
The novel coronavirus uses membrane-bound ACE2 as the receptor. The soluble form of ACE2 lacks the membrane anchor and circulates in small amounts in the blood. This soluble form may act as a competitive interceptor of SARS-CoV-2 and other coronaviruses by preventing binding of the viral particle to the surface-bound, full-length ACE2.
ACE2 fused to the Fc portion of immunoglobulin has been reported to neutralize SARS-CoV-2 in vitro. Soluble recombinant human ACE2 protein could actually be beneficial as a novel biological therapeutic to combat or limit infection progression caused by coronaviruses that utilize ACE2 as a receptor.
Human ACE2 Blocking Antibodies:
Treatment with blocking anti-ACE2 antibodies disrupts the interaction between virus and receptor, neutralizing the spread of SARS-CoV-2.
Spike S (RBD) Protein:
The CoV spike (S) protein plays the most important role in viral attachment, fusion and entry and serves as a target for development of antibodies, entry inhibitors and vaccines. The soluble recombinant RBD (receptor binding domain) of coronavirus (including SARS-CoV-2) Spike proteins exhibit significantly high binding affinity to ACE2 receptor and could block the binding by competing with the virus. Attachment of recombinant SARS-CoV-2 RBD to ACE2-expressing cells could inhibit the infection to host cells.
SARS-CoV-2 Antibodies:
SARS-CoV2 specific antibodies could cross-react with SARS-CoV-2 proteins, mainly the Spike, but also the envelope, membrane and nucleocapsid proteins, and SARS-CoV-2 induced antisera could cross-neutralize SARS-CoV-2, suggesting the potential to develop SARS-CoV-2 proteins-based vaccines for prevention of SARS-CoV-2 and SARS-CoV infections. Spike (RBD) seems to be a strong immunogenic domain of coronaviruses with a large part of the antibody immune response that is directed against this region.
LITERATURE REFERENCES:
- Angiotensin‑converting enzyme 2 (ACE2) as a SARS‑CoV‑2 receptor: molecular mechanisms and potential therapeutic target: H. Zhang, et al.; Intensive Care Med. 46, 586 (2020) (Review)
- Therapeutic options for the 2019 novel coronavirus (2019-nCoV): G. Li & E. De Clercq; Nat. Rev. Drug Discov. 19, 149 (2020) (Review)
- SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor: M. Hoffmann, et al.; Cell (Epub ahead of print) (2020)
- Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2: J.M. Penninger, et al.; Cell (Preprint) (2020)
- Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? D. Batlle, et al.; Clin. Sci. 134, 543 (2020)
COVID-19 Diagnostics
As the world is struggling to contain the novel coronavirus (COVID-19) outbreak, healthcare infrastructure and testing capacity have emerged as major issues. There is an urgent need for access to accurate and standardized diagnostics for SARS-CoV-2 (the causative agent of COVID-19). Laboratory testing for COVID-19 and the associated SARS-CoV-2 virus includes methods that detect the presence of the virus (PCR technology) and those that detect antibodies produced in response to infection. Detection of antibodies (serology) can be used both for clinical purposes and population surveillance. AdipoGen Life Sciences has an ongoing pipeline for detection of soluble ACE2, antibodies against SARS-CoV-2 and a HTS detection kit for the determination of SARS-CoV-2 blocking reagents.
LITERATURE REFERENCES:
- Detection of antibodies against SARS-CoV-2 in patients with COVID-19: Z. Du, et al.; J. Med. Virol. (Epub ahead of print) (2020)
Reagents for SARS-CoV-2/COVID-19 Research
Validated Recombinant Proteins for ACE2 & COVID-19 Research
| Product Name | PID | Source | Purity | Application/Activity |
| ACE2 (human) (rec.) | AG-40B-0192 | HEK293 cells | ≥95% (SDS-PAGE) | Soluble human ACE2 competitively inhibits SARS-CoV-2 infection. |
| ACE2 (human):Fc (human) (rec.) | CHI-B232006 | HEK293 cells | ≥90% (SDS-PAGE) | Soluble human ACE2 competitively inhibits SARS-CoV-2 infection. |
| ACE2 (mouse) (rec.) | AG-40B-0193 | HEK293 cells | ≥95% (SDS-PAGE) | Soluble mouse ACE2 negative control. |
| SARS-CoV-2 Spike Protein S1 (RBD) (rec.) (His) | CHI-B232004 | HEK 293 cells | ≥90% (SDS-PAGE) | Soluble Protein S (RBD) competitively inhibits SARS-CoV-2 infection. For drug and antibody screening applications and immunization. |
| SARS-CoV-2 Spike Protein S1 (RBD):Fc (human) (rec.) | CHI-B232003 | HEK 293 cells | ≥95% (SDS-PAGE) | Soluble Protein S (RBD) competitively inhibits SARS-CoV-2 infection. For drug and antibody screening applications and immunization. |
| SARS-CoV-2 Spike Protein S1 (RBD):Fc (human) (rec.) | AG-40B-0194 | HEK 293 cells | ≥95% (SDS-PAGE) | Soluble Protein S (RBD) competitively inhibits SARS-CoV-2 infection. For drug and antibody screening applications and immunization. |
| SARS-CoV-2 Nucleocapsid Protein (rec.) (His) | CHI-B233501 | E. coli | ≥95% (SDS-PAGE) | For drug screening applications. |
| PLpro (SARS Coronavirus) (rec.) (His) | SBB-DE0024 | E. coli | ≥95% (SDS-PAGE) | Involved in the processing of the viral polyprotein. |
| ISG15 (human) (rec.) (Rhodamine 110) | SBB-PS0002 | E. coli | ≥97% (LCMS) | PLPro substrate. Inhibits viral budding and acts as IFNγ-inducing cytokine. |
UNIQUE Human ACE2 Monoclonal Blocking Antibody
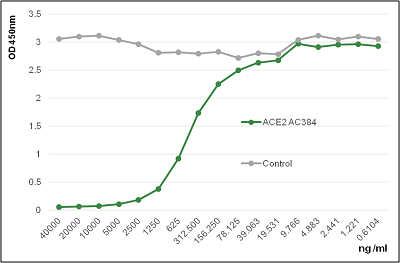
AdipoGen Life Sciences' anti-ACE2 (human), mAb (blocking) (AC384) (preservative free) (Prod. No. AG-20A-0037PF) is a monoclonal antibody that recognizes human ACE2 and works specifically in ELISA, Western Blot and Functional Application. The antibody blocks the binding of human ACE2 to the Spike protein of SARS-Cov-2.
|
Figure: Binding of ACE2 (human) to the Spike protein of SARS-CoV-2 is inhibited by the antibody anti-ACE2 (human), mAb (blocking) (AC384) (AG-20A-0037PF).
|
 |
Other Human ACE2 Antibodies
| Product Name | PID | Isotype | Applications | Species |
| anti-ACE2 (human), mAb (AC18F) | AG-20A-0032 | Mouse IgG1κ | ELISA, FACS, WB | Human |
| anti-ACE2 (human), mAb (AC18F) (Biotin) | AG-20A-0032B | Mouse IgG1κ | ELISA, FACS, WB | Human |
| anti-ACE2 (human), mAb (AC18F) (ATTO 488) | AG-20A-0032TD | Mouse IgG1κ | FACS | Human |
| anti-ACE2 (human), mAb (AC18F) (ATTO 647N) | AG-20A-0032TS | Mouse IgG1κ | FACS | Human |
| anti-ACE2 (human), mAb (AC384) | AG-20A-0037 | Mouse IgG1κ | ELISA, WB | Human |
| anti-ACE2 (human), mAb (AC384) (Biotin) | AG-20A-0037B | Mouse IgG1κ | ELISA, WB | Human |
| anti-ACE2 (human), pAb | AG-25A-0042 | Rabbit | ELISA, WB | Human |
Antiviral Compounds - Potential Small Molecule Therapeutics Against COVID-19
There are no approved drugs to treat the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection that causes coronavirus disease 2019 (COVID-19). Existing drugs, that have a known favorable safety profile, are being examined for strategies to treat the disease and fast-track a treatment plan. Several influenza and HIV drugs are currently undergoing clinical trial in coronavirus patients. The rational selection of drugs already on the market is being made based on their ability to inhibit any proteins essential for virus-receptor interaction and/or viral life cycle.
LITERATURE REFERENCES:
- Recent discovery and development of inhibitors targeting coronaviruses: T. Pillaiyar, et al.; Drug Discov. Today (Epub ahead of print) (2020)
- Rapid Identification of Potential Inhibitors of SARS-CoV-2 Main Protease by Deep Docking of 1.3 Billion Compounds: A.T. Ton, et al.; Mol. Inform. (Epub ahead of print) (2020)
- Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines: X. Liu & X.J. Wang; J. Genet. Genomics 47, 119 (2020)
- Identification of FDA Approved Drugs Targeting COVID-19 Virus by Structure-Based Drug Repositioning: P. Wang, et al.; ChemRxiv (Preprint) (2020)
- Structure of Mpro from COVID-19 virus and discovery of its inhibitors: Z. Jin, et al.; Nature (Epub ahead of print) (2020)
AdipoGen Life Sciences offers a Selection of Antiviral Small Molecules as Potential Tools for in vitro Studies of COVID-19 (not for human use).
| Product Name | PID | CAS Number | Target | Antiviral Activity |
| Amastatin . hydrochloride | AG-CP3-7003 | 100938-10-1 | Viral entry ANPEP (Aminopeptidase N) |
Viral Replication |
| Camostat mesylate | AG-CR1-3716 | 59721-29-8 | Viral entry TMPRSS2 |
Viral Replication |
| Chloroquine . diphosphate | AG-CR1-3721 | 50-63-5 | Lysosome function Zinc ionophore Immunomodulation |
Viral Replication |
| Hydroxychloroquine . sulfate | AG-CR1-3720 | 747-36-4 | Lysosome function Zinc ionophore Immunomodulation |
Viral Replication |
| Darunavir | AG-CR1-3712 | 206361-99-1 | Papain-like viral protease (PLVP) | Viral Maturation/Replication |
| Darunavir . ethanolate | AG-CR1-3724 | 635728-49-3 | Papain-like viral protease (PLVP) | Viral Maturation/Replication |
| Ebselen | AG-CR1-0031 | 60940-34-3 | Main Protease (Mpro)/3C-like Protease | Viral Transcription/Replication |
| Favipiravir | AG-CR1-3717 | 259793-96-9 | RNA-dependent RNA polymerases (RdRps) | Viral Transcription/Replication |
| Lopinavir | AG-CR1-3715 | 192725-17-0 | Coronavirus endopeptidase C30 (CEP_C30) | Viral Maturation/Replication |
| Mycophenolic acid | AG-CN2-0419 | 24280-93-1 | SARS-CoV-2 papain-like protease (PLpro) | Viral Replication |
| Niclosamide | AG-CR1-3643 | 50-65-7 | Endosome acidification | Viral Replication |
| Niclosamide . ethanolamine | AG-CR1-3644 | 1420-04-8 | Endosome acidification | Viral Replication |
| Nitazoxanide | AG-CR1-3723 | 55981-09-4 | Viral hemagglutinin Viral IE2 |
Viral Maturation/ |
| Oseltamivir . phosphate | AG-CR1-3714 | 204255-11-8 | Viral neuraminidase Release of viral particles |
Viral Replication |
| Remdesivir | AG-CR1-3713 | 1809249-37-3 | RNA-dependent RNA polymerases (RdRps) | Viral Transcription/Replication |
| AG-CR1-3722 | 1191237-69-0 | RNA-dependent RNA polymerases (RdRps) | Viral Transcription/Replication | |
| Ribavirin | AG-CR1-3719 | 36791-04-5 | RNA-dependent RNA polymerases (RdRps) RNA capping activity Viral mutation rates Immunomodulation |
Viral Transcription/Replication |
| Ritonavir | AG-CR1-3683 | 155213-67-5 | Coronavirus endopeptidase C30 (CEP_C30) | Viral Maturation/Replication |
| Ruxolitinib . phosphate salt | AG-CR1-3645 | 1092939-17-7 | JAK1/JAK2 Immunomodulation |
Viral Replication |
| Ruxolitinib (free base) | AG-CR1-3624 | 941678-49-5 | JAK1/JAK2 Immunomodulation |
Viral Replication |
| Shikonin | AG-CN2-0487 | 517-89-5 | Main Protease (Mpro)/3C-like Protease | Viral Transcription/Replication |
| Tofacitinib | AG-CR1-3625 | 477600-75-2 | JAK1/JAK3 Immunomodulation |
Viral Replication |
| Tofacitinib citrate | CDX-T0461 | 540737-29-9 | JAK1/JAK3 Immunomodulation |
Viral Replication |
| Umifenovir . HCl [Arbidol] | AG-CR1-3718 | 131707-23-8 | Viral entry Fusion into host cells |
Viral Replication |
NOT FOR HUMAN USE
Cytokine Storm and Severity of COVID-19
The most critically ill COVID-19 patients are known to undergo a cytokine storm leading to poor prognosis and need of urgent anti-inflammatory treatment/hospitalization. There are many variations on this phenomenon and they go by many names: systemic inflammatory response syndrome, macrophage activation syndrome or cytokine release syndrome (CRS).
A cytokine storm is an overproduction of immune cells and their activating compounds, the cytokines. When SARS-CoV-2 enters the lungs, it triggers an immune response, attracting immune cells to the region to attack the virus, resulting in localized inflammation. But in some patients, excessive or uncontrolled levels of cytokines are released which then activate more immune cells, resulting in hyperinflammation. The resulting lung inflammation and fluid buildup can lead to respiratory distress and can be contaminated by a secondary bacterial pneumonia. This increases the risk of mortality in patients. Cytokine storms might explain why some people have a severe reaction to coronaviruses while others only experience mild symptoms. They could also be the reason why younger people are less affected, as their immune systems are less developed and so produce lower levels of inflammation-driving cytokines. Cytokine storms are a common complication not only of COVID-19 and flu but of other respiratory diseases caused by coronaviruses such as SARS and MERS. They are also associated with non-infectious diseases such as multiple sclerosis and pancreatitis.
Therefore the diagnostic detection and treatment of cytokine storms has become an important part of rescuing severe patients. Targets like IL-1, IL-6, IL-7, IL-10, IL-18, IL-33, IFN-γ, TNF-α or many others might play an important role in cytokine release syndrome (CRS). Detection of their levels and blockage of their signaling pathways with immunomodulatory agents (biologicals, small molecules) is expected to become a new method for the treatment of severe patients.
AdipoGen Life Sciences offers a broad range of Cytokine Immunoassays as well as recombinant cytokines and blocking antibodies, which are already being successfully used to research the various cytokine storm factors involved and to characterize the immune response.
LITERATURE REFERENCES:
- COVID-19: consider cytokine storm syndromes and immunosuppression: P. Mehta, et al. ; The Lancet 395, p1033 (2020)
- The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality: C. Zhang, et al. ; Int. J. Antimicrob. Agents (Epub ahead of print) (2020)
Selected Biologicals for Cytokine Strom Research

