The original recipe reported by the Miyawaki team in 2011 termed Scale was an aqueous solution based on urea that limited because the transparency process itself can damage the structures under study.
The research team spent 5 years improving the effectiveness of the original recipe to overcome this critical challenge, and the result is ScaleS, (we called SCALEVIEW-S) a new technique with many practical applications. SCALEVIEW-S creates transparent brains with minimal tissue damage, that can handle both florescent and immunohistochemical labeling techniques, and is even effective in older animals.
The new technique creates transparent brain samples that can be stored in SCALEVIEW-S solution for more than a year without damage. Internal structures maintain their original shape and brains are firm enough to permit the micron-thick slicing necessary for more detailed analyses.
Data provided by Dr. Hiroshi Hama, Tetsushi Hoshida and Dr. Atsushi Miyawaki, Laboratory for Cell Function Dynamics, Brain Science Institute, RIKEN Biotechnological Optics Research Team, Center for Advanced Photonics, RIKEN Cooperation with Olympus
Features
- Easy-to-use
- No special equipment required
- Less damage to sample
- Compatible with IF, FP and other fluorescent labels
Application1
SCALEVIEW-S Application>
| Fixation | Clearing | Imaging | |||||||
| Perfusion fixation + post fixation 4% PFA/PBS(-) [pH 7.6 - 7.8] |
Step 1. SCALEVIEW® -S0 |
Step 2. SCALEVIEW® -S1 |
Step 3. SCALEVIEW® -S2 |
Step 4. SCALEVIEW® -S3 |
Step 5. deScale Solution |
Step 6. SCALEVIEW® -S4 |
Step 7. SCALEVIEW® -SMt |
Mounting SCALEVIEW® -SMt |
|
| Processing Temperature |
4 °C | 37 °C | 37 °C | 37 °C | 37 °C | 4°C | 37 °C | 37 °C | RT |
| Processing Time |
3 days | 30 min | 30 min | 30 min | 30 min | 3 hrs x 2 | 12 - 24 hrs | 1 hr | |
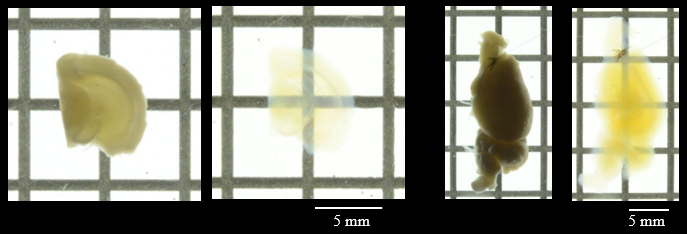
Figure 1. Transmittance images of mouse brain before and after clearing with SCALEVIEW-S Solutions.
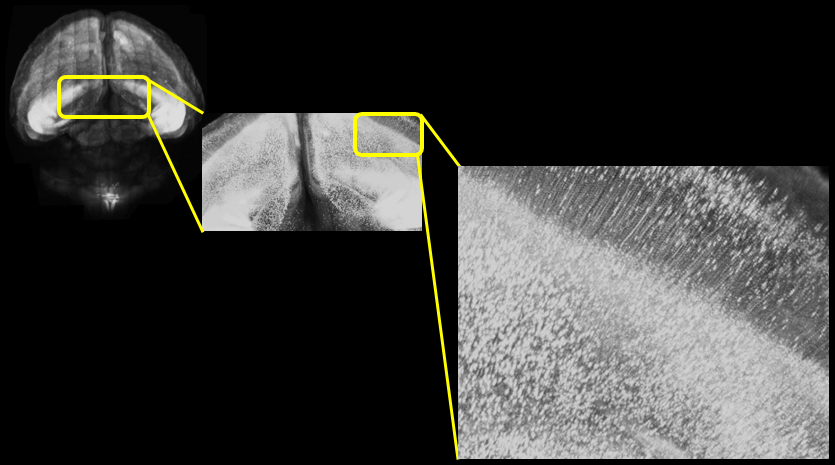
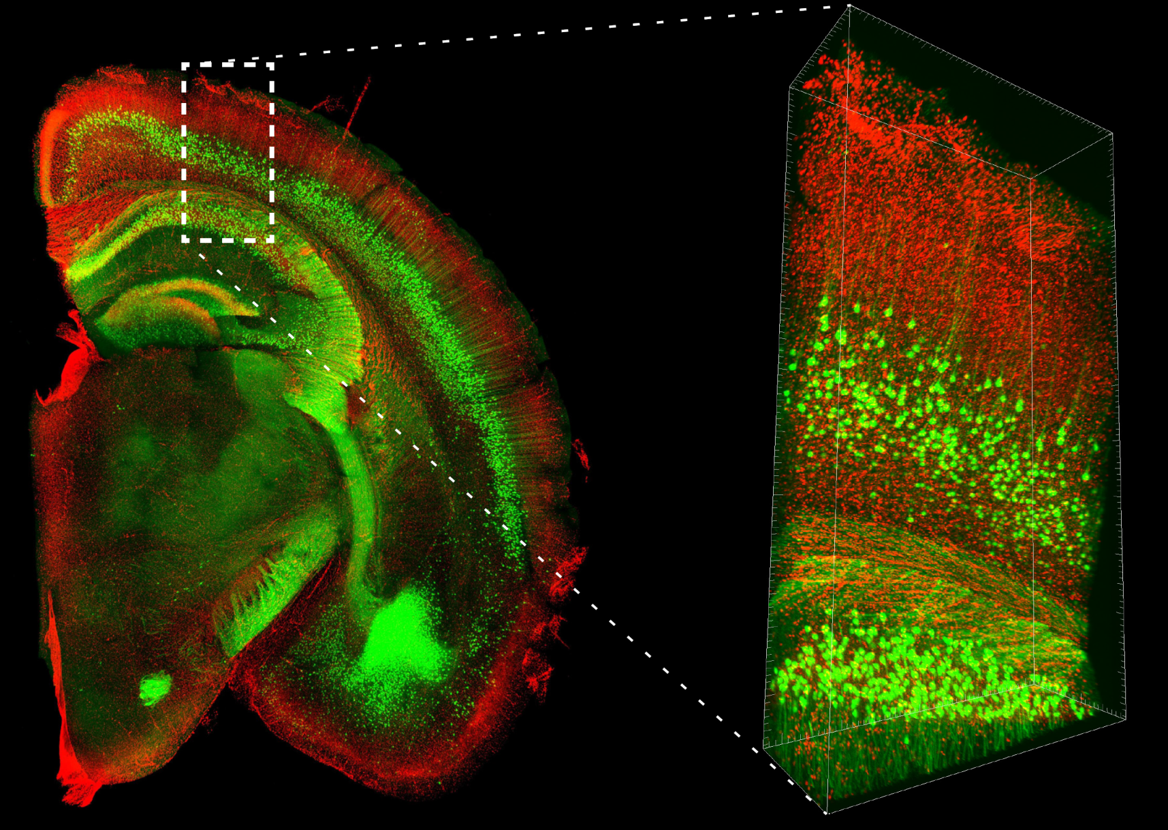
Figure 2. Two-photon microscope imaging of YFP-H line: Mouse whole brain
| Mouse | Thy1-YFP-H line, 20W, ♂ |
| Size | Whole |
| Microscope | Olympus FVMPE-RS |
| Objective lens | XLPLN10XSVMP (NA 0.6) |
| Laser | 960 nm (for YFP) |
| Image size | 512 x 512, 170 tiles, Z=8000 μm, Z Step16 μm |
Application2
SCALEVIEW-S Applications: IHC (AbScale)
| Fixation | Preprocessing | |||||
| Perfusion fixation + post fixation 4% PFA/PBS(-) [pH 7.6 - 7.8] |
Step 1. SCALEVIEW® -S0 |
Step 2. SCALEVIEW® -S1 |
Step 3. SCALEVIEW® -S2 |
Step 4. SCALEVIEW® -S3 |
Step 5. deScale Solution |
|
| Processing Temperature |
4 °C | 37 °C | 37 °C | 37 °C | 37 °C | 4°C |
| Processing Time |
3 days | 4 hrs | 4 hrs | 12 hrs | 4 hrs | 6 hrs |
| IHC | Clearing | Imaging | |||||
| Step 1. Blocking 1% Blocking Reagent (Roche)/PBS(-) |
Step 2. Staining Antibody stains |
Step 3. Wash AbScale Solution |
Step 4. Re-fixation 4% PFA/PBS(-) [pH7.6 - 7.8] |
Step 5. Wash deScale Solution |
SCALEVIEW® -S4 |
Mounting: SCALEVIEW® -S4 |
|
| Processing Temperature |
RT | 37 °C | RT | 4°C | 4°C | 37 °C | RT |
| Processing Time |
2 hrs | > 1day | 2 hrs x 1, 1 hr x 1 |
1 hr | 6 hrs | 6 - 8 hrs | |
*AbScale Solution:0.33 M Urea and 0.1 % (wt/vol) Triton X-100 in PBS(-) Solution
Iba1 (RF635: Green)
Amyloid-β (Alexa Fluor 488: Red)
Tomato lectin (Texas Red: Blue)
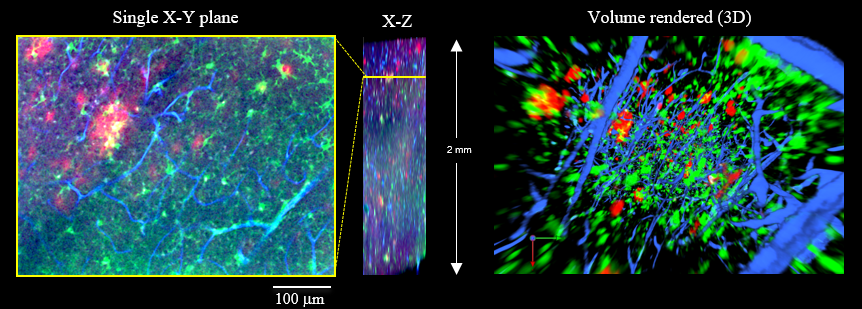
Figure 3. 3D visualization of Aβ plaques (red), microglias (green) and blood vessels (blue) from a 17-month-old AD model mouse.
| Microscope (CLSM) | Olympus FV1200 |
| Objective lens | XLPLN10XSVMP (NA 0.60) |
Application3
SCALEVIEW-S Applications: Fluorescent labels (ChemScale)
| Fixation | Preprocessing | |||||
| Perfusion fixation + post fixation 4% PFA/PBS(-) [pH 7.6 - 7.8] |
Step 1. SCALEVIEW® -S0 |
Step 2. SCALEVIEW® -A2 |
Step 3. 8M Urea Solution |
Step 4. SCALEVIEW® -A2 |
Step 5. deScale Solution |
|
| Processing Temperature |
4 °C | 37 °C | 37 °C | 37 °C | 37 °C | 4°C |
| Processing Time |
3 days | 4 hrs | 4 hrs | 12 hrs | 4 hrs | 6 hrs |
| Fluorescent labels | Clearing | Imaging | |||
| Step 1. FL labels ex:DAPI(500nM), PI(1μg/mL)/ SCALEVIEW®-A2 |
Step 2. Wash SCALEVIEW®-A2 |
Step 3. Wash deScale Solution |
SCALEVIEW® -S4 |
Mounting: SCALEVIEW® -S4 |
|
| Processing Temperature |
37 °C | 37 °C | 4 °C | 37 °C | RT |
| Processing Time |
6 -8 hrs | 2 hrs x 1, 1 hr x 1 |
3 hrs | 6 - 8 hrs | |
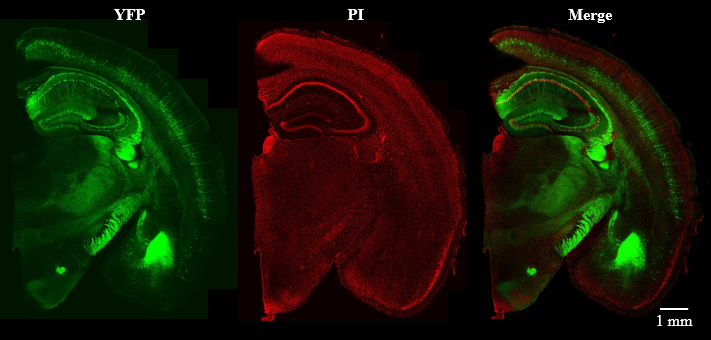
Figure 4. Confocal laser scanning microscope imaging of fluorescent labeled (PI) YFP-H line mouse brain slice (2 mm thick).
| Mouse | Thy1-YFP-H line, 42W, ♂ | Microscope (CLSM) | Olympus FV3000 (Inverted) |
| Size | Coronal Slice (2 mm) | Objective lens | UPLSAPO10x2 (NA 0.40) |
| Laser | 488 nm (for YFP), 561 nm (for PI ) |
Application4
SCALEVIEW-S Applications: Neurosphere
| Fixation | Preprocessing | |||||
| Perfusion fixation + post fixation 4% PFA/PBS(-) [pH 7.6 - 7.8] |
Step 1. SCALEVIEW® -S0 |
Step 2. SCALEVIEW® -A2 |
Step 3. 8M Urea Solution |
Step 4. SCALEVIEW® -A2 |
Step 5. deScale Solution |
|
| Processing Temperature |
RT | 37 °C | 37 °C | 37 °C | 37 °C | 4°C |
| Processing Time |
1 hr | 4 hrs | 4 hrs | 12 hrs | 4 hrs | 6 hrs |
| IHC | Clearing | Imaging | |||||
| Step 1 Blocking 1% Blocking Reagent (Roche)/PBS(-) |
Step 2. Staining Antibody stains |
Step 3. Wash deScale Solution |
Step 4. Re-fixation 4% PFA/PBS(-) [pH7.6 - 7.8] |
Step 5. Wash deScale Solution |
SCALEVIEW®-S4 | Mounting: SCALEVIEW®-S4 |
|
| Processing Temperature |
RT | 37 °C | RT | RT | 4°C | 37 °C | RT |
| Processing Time |
2 hrs | > 1day | 2 hrs x 1, 1 hr x 1 |
1 hr | 3 hrs | 4 hrs | |
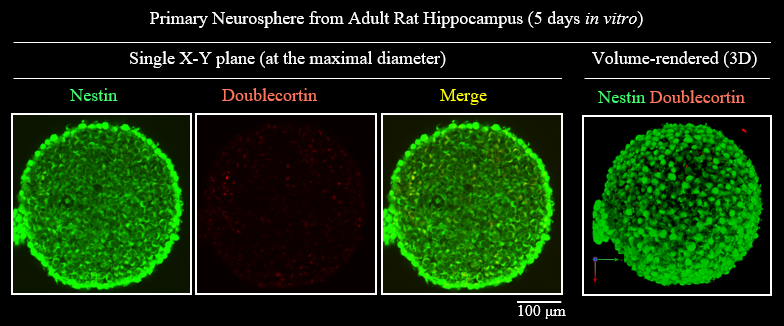
Figure5. 3D visualization of Neurosphere>/p>
| Microscope (CLSM) | Olympus FV1000 |
| Objective lens |
UMPLFLN10XW (NA 0.3) |
References
- Hama,H.et al. : Nature Neuroscience 14, 1481(2011).
- Hama,H.et al. : Nature Neuroscience 18, 1518(2015).
- Hama H, et al. : Protocol Exchange (2016), doi:10.1038/protex.2016.019
- Molly E, Boutin, et al :Scientific Reports, 8, 11135 (2018).
